The Clinical Development of Sofosbuvir/Velpatasvir/Voxilaprevir (SOF/VEL/VOX, Vosevi®) | SpringerLink
Informe de Posicionamiento Terapéutico de sofosbuvir/velpatasvir/voxilaprevir (Vosevi®) en hepatitis C

PDF) Sofosbuvir/Velpatasvir/Voxilaprevir for Patients with HCV Who Previously Received a Sofosbuvir/Velpatasvir-Containing Regimen: Results from a Retreatment Study
1 Resumen del Congreso EASL 2019 Aspectos microbiológicos relacionados con el diagnóstico y tratamiento de las hepatitis vira
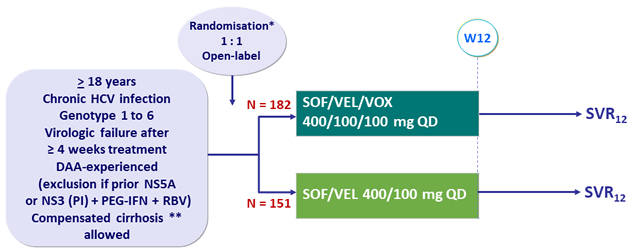
SOF/VEL/VOX for 12 Weeks in NS5A-Inhibitor-Experienced HCV-Infected Patients: Results of the Deferred Treatment Group in the Phase 3 POLARIS-1 Study

Prevalencia de las potenciales interacciones medicamentosas entre los antivirales de acción directa pangenotípicos y la medicación concomitante asociada a los pacientes con infección del virus de la hepatitis C crónica en España
HCV-Trials.com : A regularly updated website with all most recent clinical trials data in HCV infection

The Clinical Development of Sofosbuvir/Velpatasvir/Voxilaprevir (SOF/VEL/VOX, Vosevi®) | SpringerLink

PDF) Efficacy of sofosbuvir/velpatasvir/voxilaprevir in direct-acting antiviral experienced patients with hepatitis C virus

Effectiveness and safety of sofosbuvir/velpatasvir/voxilaprevir in patients with chronic hepatitis C previously treated with DAAs - ScienceDirect
HCV-Trials.com : A regularly updated website with all most recent clinical trials data in HCV infection

Sofosbuvir/Velpatasvir/Voxilaprevir (SOF/VEL/VOX) for Prior Treartment Failures with Glecaprevir/Pibrentasvir (G/P) in Chronic Hepatitis C Infection
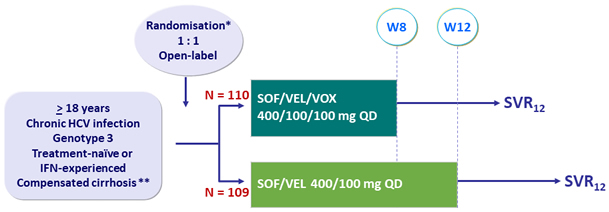
A Randomized Phase 3 Trial of Sofosbuvir/Velpatasvir/Voxilaprevir for 8 Weeks Compared to Sofosbuvir/Velpatasvir for 12 Weeks in